News
Radiology Roundup of New FDA Clearances: January 11 — January 17 | Diagnostic Imaging
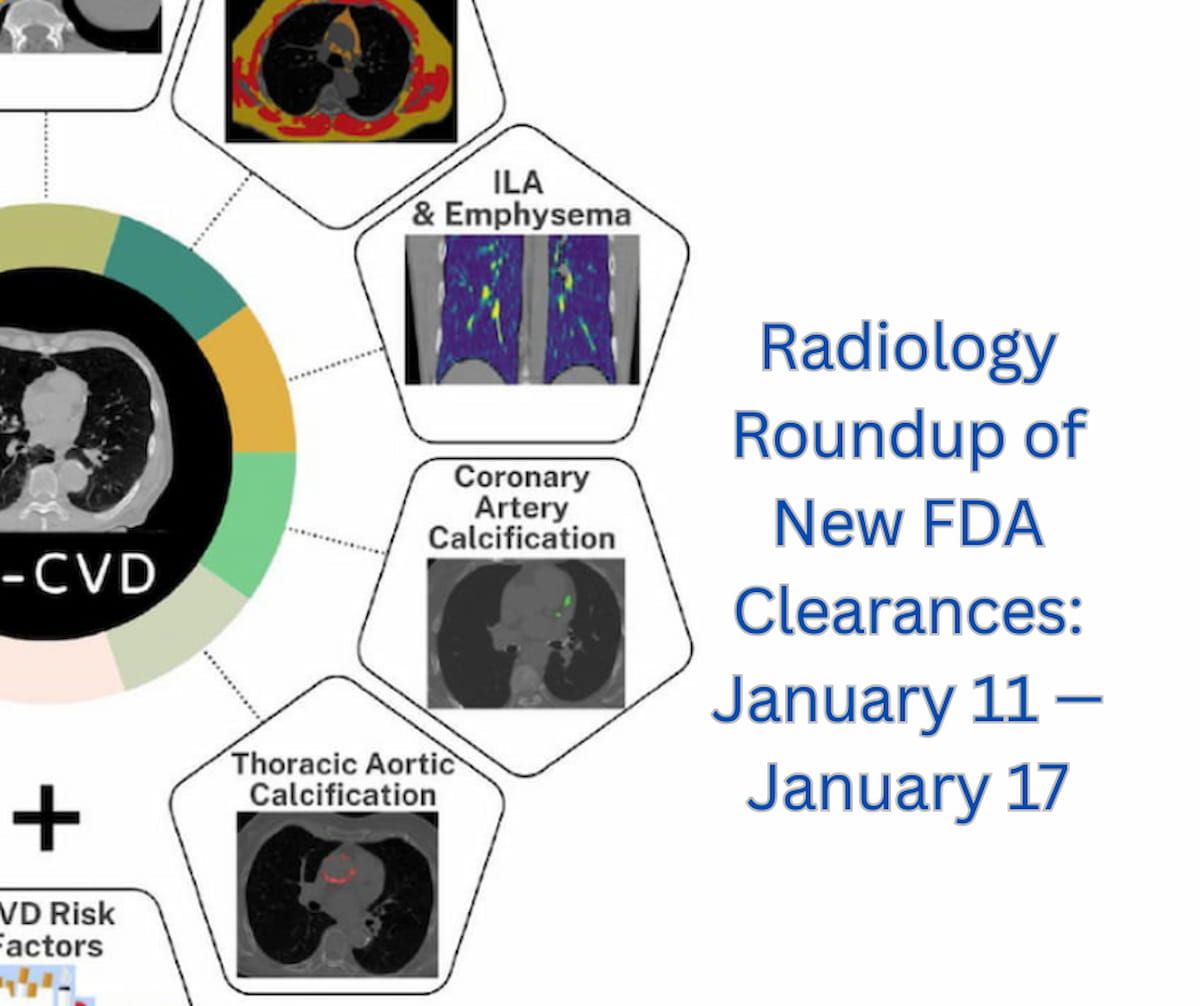 2+ hour ago (107+ words) Radiology Roundup of New FDA Clearances: January 11 " January 17diagnosticimaging.com Catch up on a variety of new FDA clearances in radiology from the past week. From a new point-of-care ultrasound platform and a breast biopsy system that can be utilized across…...
2+ hour ago (107+ words) Radiology Roundup of New FDA Clearances: January 11 " January 17diagnosticimaging.com Catch up on a variety of new FDA clearances in radiology from the past week. From a new point-of-care ultrasound platform and a breast biopsy system that can be utilized across…...
Diagnostic Imaging's Weekly Scan: January 11 — January 17 | Diagnostic Imaging
 14+ hour, 30+ min ago (249+ words) Catch up on the top radiology content of the past week. Welcome to'Diagnostic Imaging's Weekly Scan, which offers an opportunity to catch up on the most well-viewed radiology content of the past week. In a new retrospective breast MRI study…...
14+ hour, 30+ min ago (249+ words) Catch up on the top radiology content of the past week. Welcome to'Diagnostic Imaging's Weekly Scan, which offers an opportunity to catch up on the most well-viewed radiology content of the past week. In a new retrospective breast MRI study…...
Multi-Modality Breast Biopsy System Garners FDA Clearance | Diagnostic Imaging
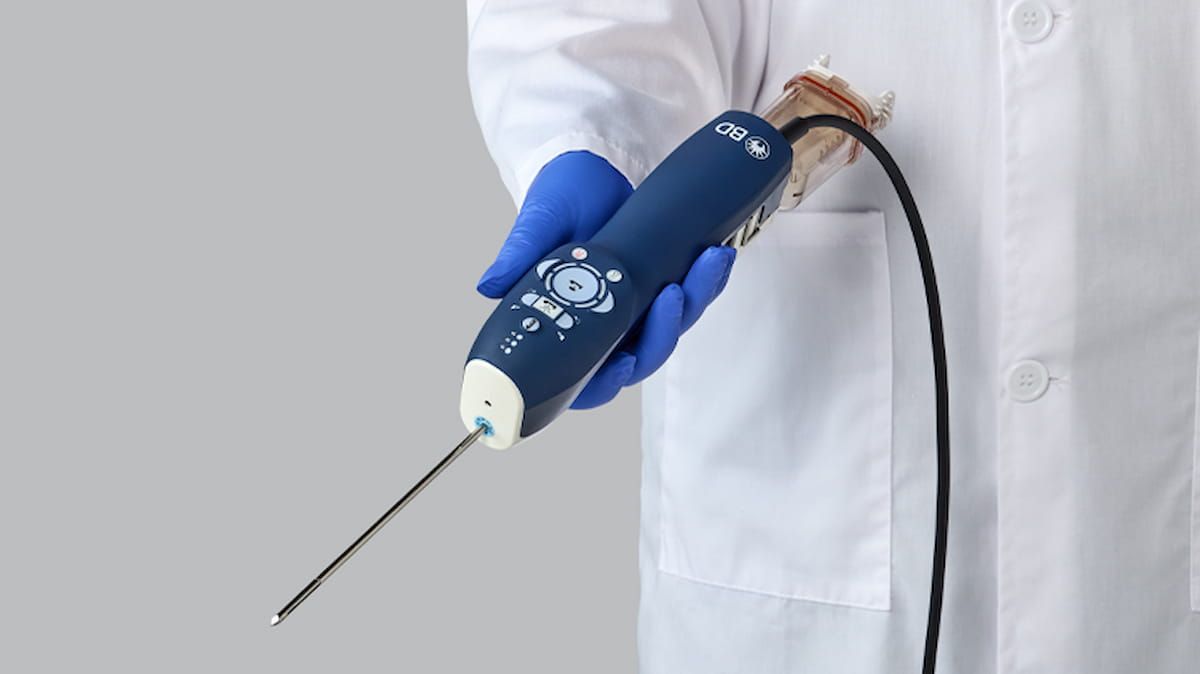 1+ day, 20+ hour ago (203+ words) Offering an echogenic cutting cannula and enhanced sampling capability, the EnCor EnCompass Breast Biopsy and Tissue Removal System reportedly enables biopsy procedures across different breast imaging platforms. The Food and Drug Administration has granted 510(k) clearance for the EnCor EnCompass Breast…...
1+ day, 20+ hour ago (203+ words) Offering an echogenic cutting cannula and enhanced sampling capability, the EnCor EnCompass Breast Biopsy and Tissue Removal System reportedly enables biopsy procedures across different breast imaging platforms. The Food and Drug Administration has granted 510(k) clearance for the EnCor EnCompass Breast…...
FDA Clears Multimodality Tumor Marker for Breast Cancer Imaging | Diagnostic Imaging
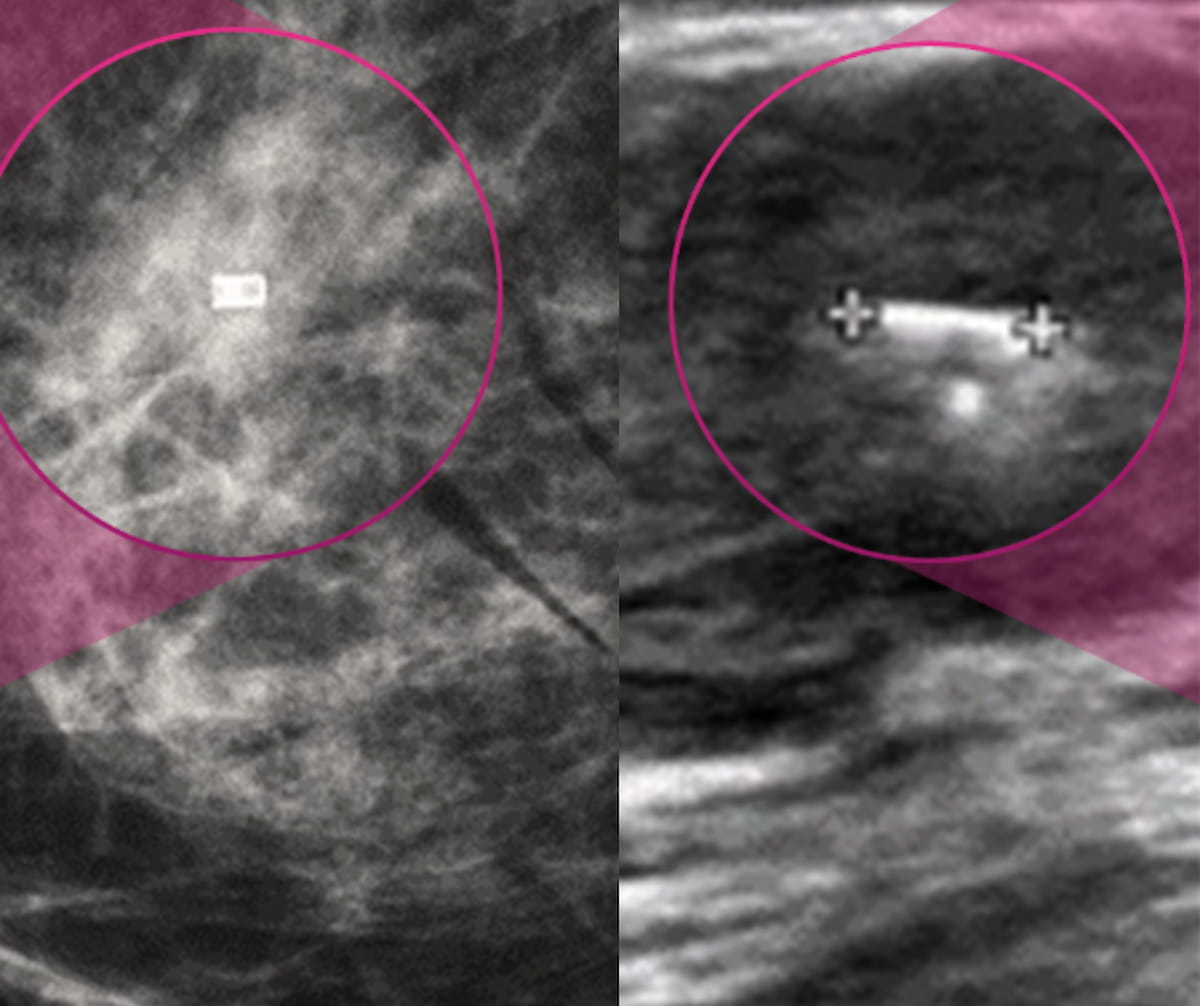 2+ day, 16+ hour ago (285+ words) Offering a biocompatible, non-metallic alternative to metallic tumor markers, the VM1 tumor marker reportedly offers artifact-free visibility across a variety of breast imaging including mammography, ultrasound and MRI. The Food and Drug Administration (FDA) has granted 510(k) clearance for the emerging non-metallic…...
2+ day, 16+ hour ago (285+ words) Offering a biocompatible, non-metallic alternative to metallic tumor markers, the VM1 tumor marker reportedly offers artifact-free visibility across a variety of breast imaging including mammography, ultrasound and MRI. The Food and Drug Administration (FDA) has granted 510(k) clearance for the emerging non-metallic…...
A Closer Look at Automated LLM Protocoling for Abdominal and Pelvic CT | Diagnostic Imaging
 2+ day, 19+ hour ago (372+ words) In a recent interview, Rajesh Bhayana, M.D., discussed pertinent findings from a new study examining the use of the large language model GPT-4o in facilitating protocoling for abdominal and pelvic CT exams. As an abdominal radiologist, Rajesh Bhayana, M.D., said image protocoling…...
2+ day, 19+ hour ago (372+ words) In a recent interview, Rajesh Bhayana, M.D., discussed pertinent findings from a new study examining the use of the large language model GPT-4o in facilitating protocoling for abdominal and pelvic CT exams. As an abdominal radiologist, Rajesh Bhayana, M.D., said image protocoling…...
Challenges with Diversity and Residency Attrition Rates in Interventional Radiology | Diagnostic Imaging
 3+ day, 22+ hour ago (281+ words) In a recent interview, Mina Makary, M.D., discussed recent research examining diversity within interventional radiology (IR), attrition rates in IR residency programs and potential strategies to bolster recruitment and retention. Based on data from the annual Association of American Medical Colleges…...
3+ day, 22+ hour ago (281+ words) In a recent interview, Mina Makary, M.D., discussed recent research examining diversity within interventional radiology (IR), attrition rates in IR residency programs and potential strategies to bolster recruitment and retention. Based on data from the annual Association of American Medical Colleges…...
FDA Clears Emerging LU800 Handheld Ultrasound Device | Diagnostic Imaging
 4+ day, 6+ hour ago (252+ words) Offering compact versatility for an array of clinical settings, the ASUS Handheld Ultrasound LU800 is reportedly the first handheld device to be supported across Android, Chrome, iOS and Windows operating systems. The Food and Drug Administration (FDA) has granted 510(k) clearance for…...
4+ day, 6+ hour ago (252+ words) Offering compact versatility for an array of clinical settings, the ASUS Handheld Ultrasound LU800 is reportedly the first handheld device to be supported across Android, Chrome, iOS and Windows operating systems. The Food and Drug Administration (FDA) has granted 510(k) clearance for…...
Another Take on the OLA for Maintaining Certification with the American Board of Radiology | Diagnostic Imaging
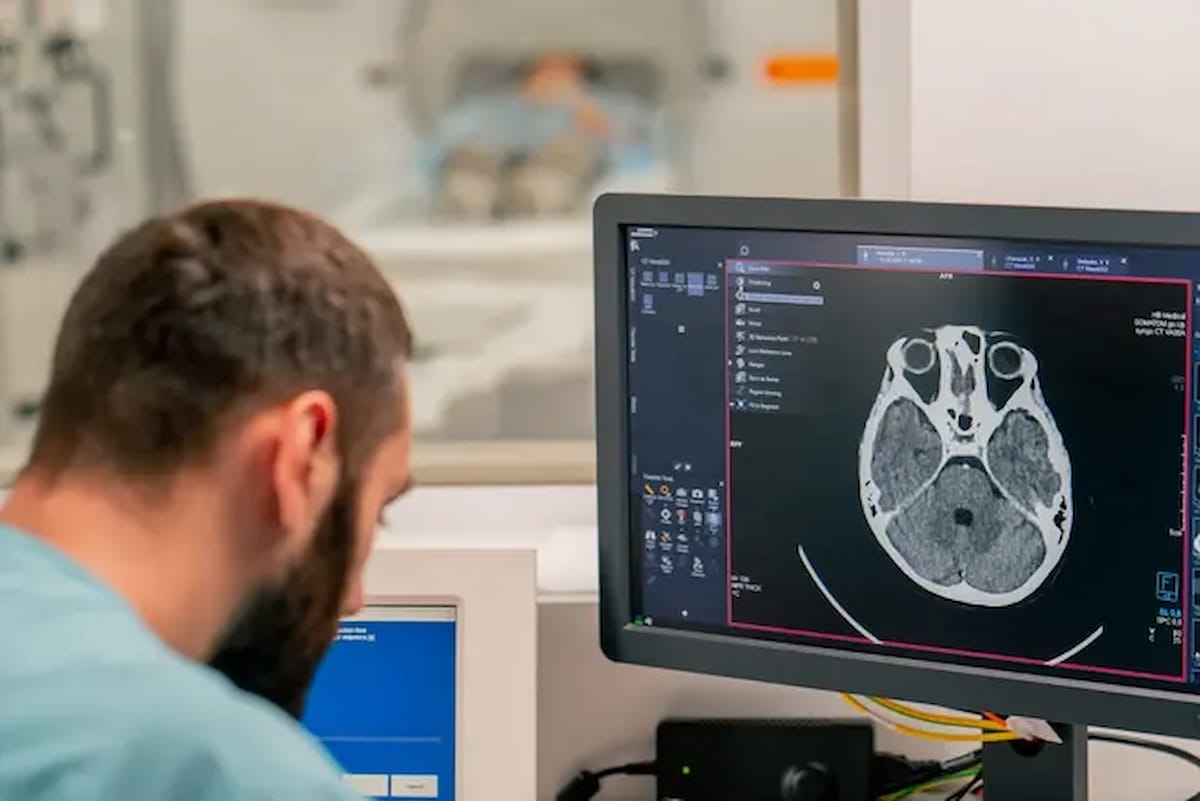 6+ day, 1+ hour ago (715+ words) Is the current OLA process a viable alternative for assessing our skills? A small cascade of emails hits my inbox every time a new month begins. A slightly bigger one happens when the year turns over. The ABR's other annual…...
6+ day, 1+ hour ago (715+ words) Is the current OLA process a viable alternative for assessing our skills? A small cascade of emails hits my inbox every time a new month begins. A slightly bigger one happens when the year turns over. The ABR's other annual…...
FDA Clears AI Software for Opportunistic Multisystem Computed Tomography Screening | Diagnostic Imaging
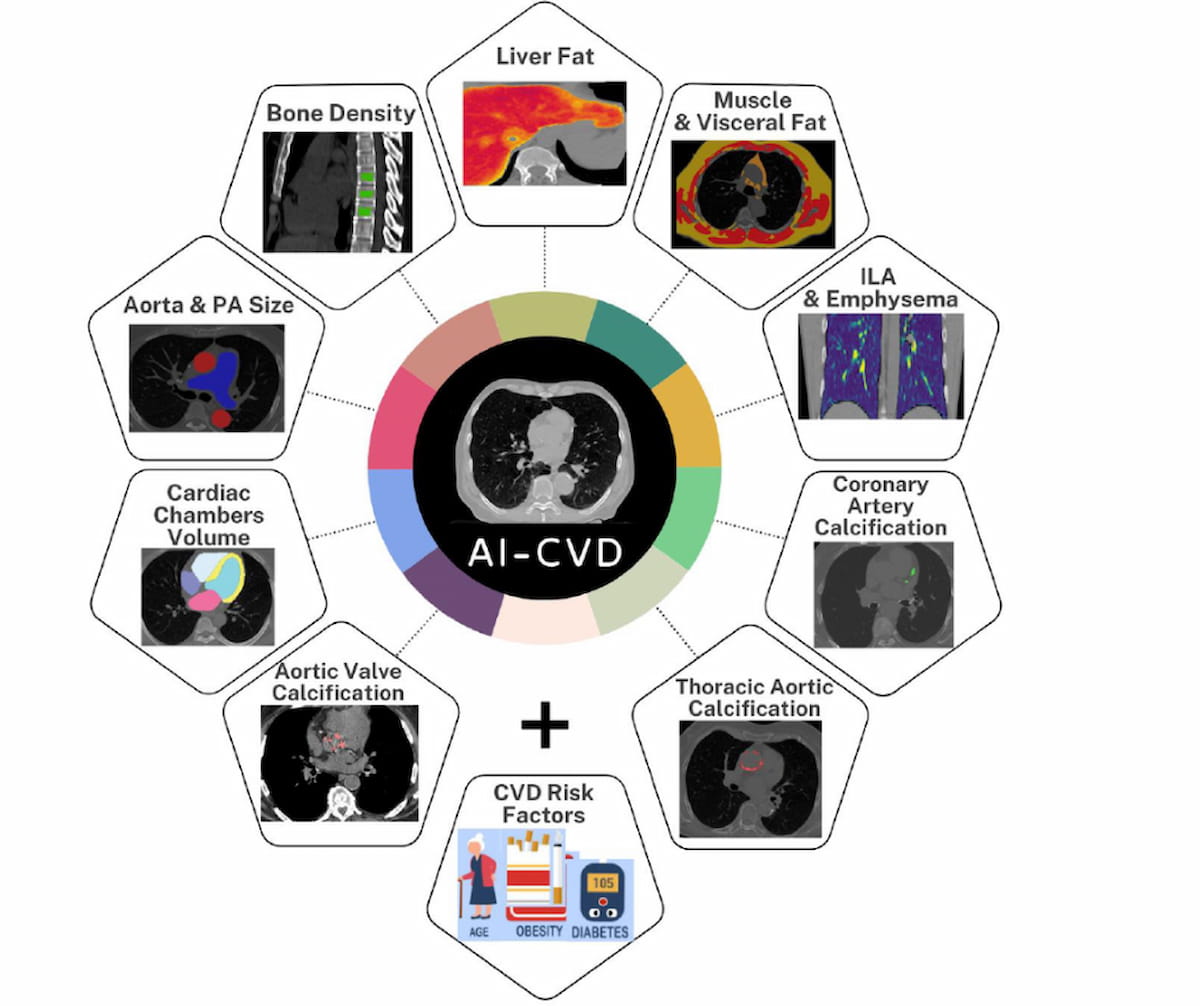 6+ day, 8+ hour ago (349+ words) The AI-CVD software reportedly combines automated quantitative measurements for cardiovascular assessments such as coronary artery calcium (CAC) scoring and epicardial fat volumetry with lung attenuation and liver attenuation analysis in one CT-based AI platform. The Food and Drug Administration has…...
6+ day, 8+ hour ago (349+ words) The AI-CVD software reportedly combines automated quantitative measurements for cardiovascular assessments such as coronary artery calcium (CAC) scoring and epicardial fat volumetry with lung attenuation and liver attenuation analysis in one CT-based AI platform. The Food and Drug Administration has…...
Emphasizing a Proactive Embrace of AI in Radiology | Diagnostic Imaging
 1+ week, 15+ hour ago (44+ words) Emphasizing a Proactive Embrace of AI in Radiology'diagnosticimaging.com Trending on Diagnostic Imaging FDA Clears 1.5T MRI Platform with Helium-Free Technology FDA Issues Expanded Clearance for UltraSight's Cardiac Ultrasound Echo Stewardship Program Could LLMs Enhance Protocoling Efficiency for Abdominal and Pelvic…...
1+ week, 15+ hour ago (44+ words) Emphasizing a Proactive Embrace of AI in Radiology'diagnosticimaging.com Trending on Diagnostic Imaging FDA Clears 1.5T MRI Platform with Helium-Free Technology FDA Issues Expanded Clearance for UltraSight's Cardiac Ultrasound Echo Stewardship Program Could LLMs Enhance Protocoling Efficiency for Abdominal and Pelvic…...